As you may know, Northwest Integrative Medicine has been offering monoclonal antibody treatments for the prevention and treatment of COVID-19. The use of these therapies for prevention are NOT to replace our highly effective and safe vaccinations that have been developed. In fact, if you haven’t already, talk with your healthcare provider if you are eligible for a COVID-19 vaccine or booster dose.
Before we get into the nuts and bolts of the therapy, first we need some background context. Currently, the FDA has provided Emergency Use Authorization (EUA) for a medication known as REGEN-COV®, which, under the Federal Food, Drug, and Cosmetic Act, allows the FDA to “Authorize unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions…when certain criteria are met, including there are no adequate, approved, and available alternatives”, a long way of stating that there’s a big emergency going on and from what we do know, this is currently the best and most safe option we can provide at the moment.
WHO is eligible for the therapy?
In the state of Oregon, you or someone you know may qualify for therapy if you meet the following criteria:
- At least 12 years of age AND weigh ≥ 88 pounds
- Have been exposed to COVID-19 or at high risk of exposure OR
- Have tested positive for COVID-19 and are within 10 days of symptoms
- Have tested positive for COVID-19 and have mild to moderate symptoms
- Have tested positive for COVID-19 and do not require oxygen therapy
- Have tested positive for COVID-19 and are at high risk for developing severe symptoms
- Not hospitalized
WHAT is REGEN-COV®?
- Combination of 2 antibodies given as one medication: Casirivimab (Kas-eer-iv-im-ab) and Imdevimab
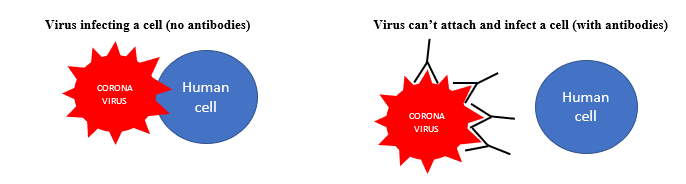
- These medications bind to the Corona-19 virus at a particular location preventing its ability to attach and enter our cells
WHERE can I get more information?
In the state of Oregon, the Oregon Health Authority has been helping to provide information as to the number of sites receiving an allotted number of REGEN-COV® therapy.
More information regarding monoclonal antibodies for COVID-19, more generally, can be also be accessed at the Combat COVID website.
WHY should I consider getting this therapy?
Two reasons:
- You were exposed to confirmed case, and you want to reduce the risk of also testing positive and/or developing symptoms
- You tested positive and want to reduce the risk of symptoms progressing, needing to be hospitalized, or decrease the duration of your symptoms
HOW effective is the treatment?
- Effective against 6 variants including the delta variant. Currently we do NOT know if it is effective against the newest variant, Omicron
- Itreduces the risk of hospitalization by 70% in those who test positive
- Itreduces the risk of testing positive and developing symptoms by 81% in those who were exposed to someone who tested positive to COVID-19
- Itreduces duration of infection by 1 week
- Itreduces symptoms by 4 days in those who tested positive
HOW is it administered?
Two ways:
- IV infusion (not offered at NWIM) – More appropriate for treatment
- Subcutaneous injection (fancy term for under the skin) – 1 injections in 4 different sites of the body – More appropriate for prevention, but can be used for treatment
HOW safe is the treatment?
Majority of safety data comes from two trials of several thousand individuals. For comparison, we have SIGNIFICANTLY more safety data available from the COVID-19 vaccines than we do with the antibody treatments. More safety data is likely needed but from what IS available, the most common side effects from injections are headaches and local reactions (arm pain, swelling, redness). In one clinical trial, only 4% of participants had local reactions and less than 2% had headaches, fewer than 1% had serious adverse reactions. Interestingly, more individuals who got “fake” medication had more side effects than those who got the actual medication. IV infusions were also well tolerated by most individuals studied in another clinical trial.
Interested in discussing this topic in more detail? Want to know if you qualify for treatment? Check out the entire NWIM team and schedule your appointment today!
References:
- Emergency Use Authorization. U.S. Food & Drug Administration. Updated 12/10/2021. Accessed 12/10/2021. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
- COVID-19 Response. Oregon Health Authority. Accessed 12/10/2021. https://www.oregon.gov/oha/covid19/Pages/monoclonal-antibody-therapy.aspx
- Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19. N Engl J Med. 2021;385(23)
- O’Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19. N Engl J Med. 2021;385(13):1184-1195.
